Life is a complex set of reactions that take place inside living organisms. A lot of these reactions need a little help in the form of catalysts. One of the biggest catalytic reactions is via enzymes. They are extremely specific and usually do one job well. They are also very dependent on temperature. Certain enzymes work well in a certain temperature range. Their efficacy decreases outside that range. The theory to this date has been that high temperature causes enzymes to follow Arrhenius behavior (i.e. things get faster as it gets hotter, or with a lower energy barrier) and break their bonds, thus unfolding into a different shape and losing their efficacy.
Enzymatic Enigma Explained
Scientists at the University of Bristol, Adrian Mulholland and Dr. Marc Van der Kamp, as well as Vic Arcus out of the University of Waikato in New Zealand showed in Biochemistry that the heat capacity (the number of heat units needed to raise the temperature of the enzyme by one degree, usually denoted by ∆Cp) changes during a reaction as the enzymes tighten up. So there’s no actual breaking of bonds or loss of shape. The level of tightening of the enzyme structure is what determines at what temperature it will work best. The information from this can be used to design better biocatalysts in industrial processes, for example in the production of life saving drugs.
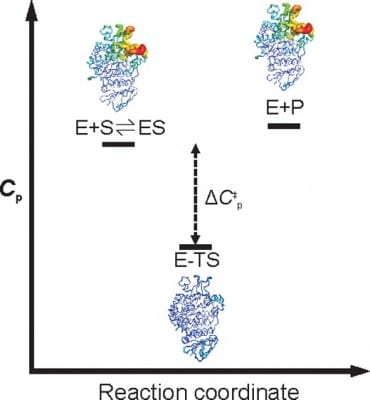
So now that the theory that the breaking down of the molecular shape of an enzyme causes it to lose efficacy is being challenged, what exactly makes them less active if the shapes are indeed similar? The new research shows that the heat capacity both explains and predicts the dependence of enzymes upon temperatures. The investigators coined the phrase ‘Macromolecular Rate Theory (MMRT)’ and they posit that it would apply to all enzymes, and thus could predict the optimal temperature (Topt) for each enzyme to have maximum efficacy. It would also explain why the more complex the reaction, the bigger the enzyme (or the more difficult the chemistry behind it).
[button link=”http://pubs.acs.org/doi/abs/10.1021/acs.biochem.5b01094″ icon=”fa-external-link” side=”left” target=”blank” color=”285b5e” textcolor=”ffffff”]Source: Biochemistry [/button]